The news for hydrogen fuel cell vehicles has been something of a mixed bag lately. Last month Honda's Clarity became the first ever fuel cell electric vehicle to make the Top Ten Engines list from leading industry researcher WardsAuto. In the same month, though, the headlines were full of dire news suggesting that interest in fuel cell EVs is about to fall off a cliff.
So, what to make of all this? One challenge for hydrogen fuel cell EVs is the fuel itself. Natural gas is the main source for hydrogen, and that's a significant handicap. However, sustainable sources are beginning to emerge. In the latest development, a team of researchers at Columbia Engineering has introduced a concept for large scale, low cost hydrogen production using seawater as a source.
Why floating seawater-to-hydrogen?
The basic idea behind the new system is electrolysis, which refers to "splitting" water with an electrical current and a catalyst.
Until recent years pure water was required, and the electricity would typically lean on conventional sources including coal, natural gas and nuclear.
For the past several years, though, researchers have been deploying solar and wind energy to provide a current for splitting seawater, sewage, and other impure sources.
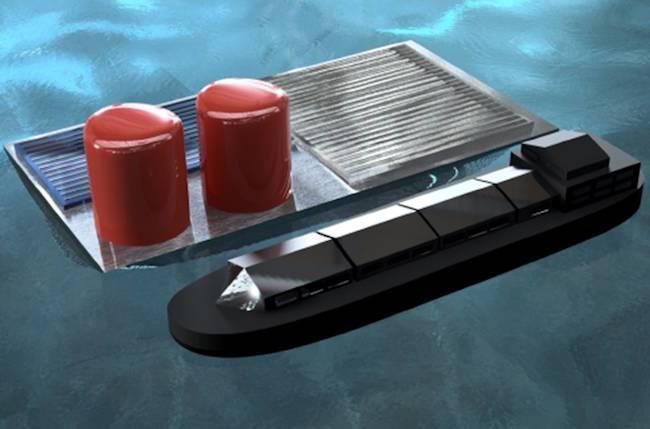
Once you have seawater-to-hydrogen in hand, the next logical step is to develop a system that can float in the seawater itself.
There are several advantages. The most obvious one is that the facility is located at the sources, so no energy (pumps, etc.) is required to transport the seawater to a watersplitting facility.
In the U.S., high populations are concentrated along coastlines, so a floating facility would generate fuel fairly close to a high demand market. That also cuts down on energy related to transportation.
A floating facility would also help resolve land use issues for crowded coastal areas.
Because seawater is the water source, there is also no competition for water resources needed for agriculture, domestic or industrial uses (or, for that matter, environmental conservation).
Getting the H out of H2O -- with bubbles!
The Columbia Engineering system demonstrates all three advantages, with the added -- and essential -- benefit of low production costs.
The new system is being shepherded by Daniel Esposito, an assistant professor of chemical engineering who has been focusing his research team on solar-powered electrolysis.
Here's the rundown from Columbia Engineering:
Esposito’s team has now developed a novel photovoltaic-powered electrolysis device that can operate as a stand-alone platform that floats on open water. His floating PV-electrolyzer can be thought of as a “solar fuels rig” that bears some resemblance to deep-sea oil rigs, except that it would produce hydrogen fuel from sunlight and water instead of extracting petroleum from beneath the sea floor.
To cut costs down to the bone, the research team zeroed in on the membrane required by the latest generation of high efficiency electrolyzers. The membrane is needed to keep the hydrogen and oxygen gasses apart.
The Columbia Engineering solution was to jettison the membrane altogether. Instead, the new device leverages the buoyancy of bubbles.
The basic concept is simple. The electrolyzer, sans membrane, is submersed in seawater. Once an electrical current is applied, the device generates bubbles of hydrogen and oxygen gas. Then the magic happens:
The generated H2 bubbles are harvested within the interior of the device as they float upwards, while O2 bubbles are allowed to vent to the atmosphere.
The devil, of course, is in the details. The electrolyzer catalyst is a key factor, so one challenge was to design a new catalyst that separates the hydrogen and oxygen bubbles as they form.
Without the need for a membrane, the system takes a shortcut around one of the major durability challenges for submerging devices in seawater.
As for the electrical current, the floating rig is festooned with photovoltaic cells, providing an ample supply of electricity during daylight hours. Add energy storage, and the system has the potential to operate 24/7.
Biomimicry to the rescue
If all this is beginning to sound like photosynthesis, you're on to something. Plants deploy sunlight to produce the building blocks for growth. Natural photosynthesis evolved over millions of years. The new solar-powered hydrogen system, and others like it, represent the application of science to duplicate that same creative power within a generation.
First author on the new study Jack Davis explains:
These solar fuels generators are essentially artificial photosynthesis systems, doing the same thing that plants do with photosynthesis, so our device may open up all kinds of opportunities to generate clean, renewable energy.
So far the new electrolyzer has passed its workout in "artificial" seawater -- basically, a tailor-made salty solution. Next steps include immersing the device in actual seawater, where all sorts of unexpected variables could pop up.
Modular-izing the design is another goal, which would help with scalability and construction costs.
For more details about the Columbia study, look up “Floating Membraneless PV-Electrolyzer Based on Buoyancy-Driven Product Separation” in the Journal of Hydrogen Energy. For the record, in addition to Esposito and Jack Davis, the study authors are Jonathan Davis, Ji Qi, Xinran Fan and Justin Bui.
To keep up with the latest from Esposito's research team, check out the Solar Fuels Engineering Laboratory at Columbia Engineering.
Image credit: Justin Bui / Columbia Engineering.

Tina writes frequently for TriplePundit and other websites, with a focus on military, government and corporate sustainability, clean tech research and emerging energy technologies. She is a former Deputy Director of Public Affairs of the New York City Department of Environmental Protection, and author of books and articles on recycling and other conservation themes.














